|
Getting your Trinity Audio player ready...
|
OWN VOICE. ~ InPerspective by Bruce Laird —
What’s Going On Here?
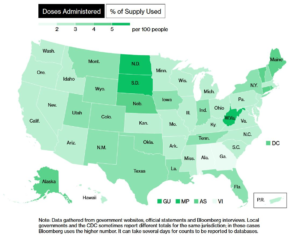
If you want to track progress of shipments to, and administration of doses by different states, Bloomberg has put up a good vaccination tracker: “More Than xxx Million Shots Given: Covid-19 Vaccine Tracker.” at Bloomberg.com. Example at right.
Considering that North and South Dakota have been leading the U.S. in most COVID deaths per capita since 1 Jul 2020 [chart below], it is a relief to see that as of 7 Jan 2021, the Dakotas are in 2nd and 3rd place nationally in terms of most doses given per 100 people. West Virginia is leading (4.13 per 100, 74k doses); followed by South Dakota (3.77/100, 33k doses); and N. Dakota (3.58/100, 27k doses).
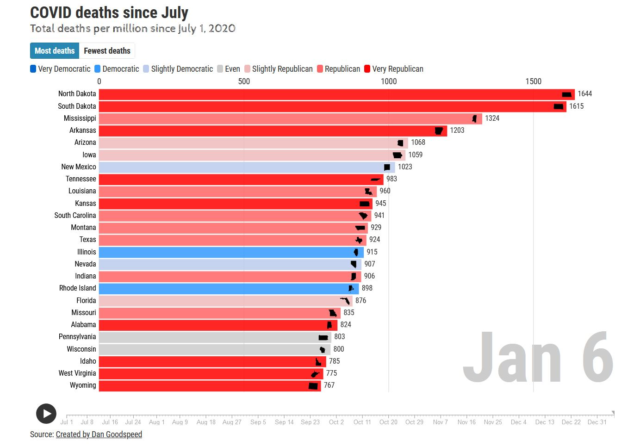
You can track recent trends in cases and deaths in an interactive “horse race” at Dan Goodspeed’s website:
Escape mutants and delayed booster doses
Helen Branswell has written another excellent article, this time on the debate over whether/how to stretch the current limited supply of doses to cover the maximum number of people in the early months of vaccination programs. See: “Britain takes a gamble with Covid-19 vaccines, upping the stakes for the rest of us.” statnews, 4 Jan 2020.
From Helen’s article:
In recent days, the British have said they will stretch out the interval between the administration of the two doses required for Covid-19 vaccines already in use — potentially to as long as three months, instead of the recommended three or four weeks. And they have said they will permit the first dose and second dose for any one person to be from different vaccine manufacturers, if the matching vaccine is not available.
The moves are based on small slices of evidence mined from “subsets of subsets” of participants in clinical trials, as one expert described it for STAT, and on general principles of vaccinology rather than on actual research into the specific vaccines being used. If the efforts succeed, the world will have learned a great deal. If they fail, the world will also have gained important information, though some fear it could come at a high cost.
Paul Bieniasz of Rockefeller University is one of those who is watching the evolving situation in Britain with dread. A retrovirologist who turned from HIV research to work on SARS-2, Bieniasz is studying how the virus acquires mutations that allow it to evade the protective antibodies people develop when they have contracted Covid-19, or when they have been vaccinated against it.
“My concern, as a virologist, is that if you wanted to make a vaccine-resistant strain, what you would do is to build a cohort of partially immunized individuals in the teeth of a highly prevalent viral infection,” Bieniasz told STAT. Even rolling out the vaccine at all when there is so much transmission occurring is far from ideal, he said, suggesting it would have been safer to beat down the amount of virus in circulation before beginning the vaccine deployment.
“You are essentially maximizing the opportunity for the virus to learn about the human immune system. Learn about antibodies. Learn how to evade them,” he said.
Here is Bieniasz’s sarcastic twitter post, “Musings of an anonymous, pissed off virologist”, twitter, 1 Jan 2021…. Which argues that spreading out weak doses is the best way to teach the virus how to successfully mutate (which it seems to be doing very well on its own).
Musings of an anonymous, pissed off virologist. pic.twitter.com/IVU1COZPof
— Paul Bieniasz (@PaulBieniasz) January 2, 2021
On the other side of this argument, the argument for spreading the first doses as widely as possible, at the expense of delayed booster shots, is Dr. John Campbell, a retired Oxford instructor. Since last spring, he has produced a series of very informative and watchable videos on youtube. Here is his take on the AZN vaccine, and spreading the doses:
https://www.youtube.com/watch?v=8Pj4_aK-j8I
It appears that Dr. Campbell’s claims about spacing out the doses are unique to the AZN vaccine. He points out that in a small number of cases in AZN’s phase 3 trials outside the U.S., the AZN booster shot was administered 6-12 weeks after the first shot. His claim (with no data): there were fewer “serious” cases, as measured by reduced hospitalizations – after just one shot. He says the data for this have not yet been made publicly available; so only the U.K. Public Health oversight org has seen them. Hmm.
Helen Branswell offers slightly more detail about the delayed AZN booster in her statnews article, but she does not include claims like Campbell’s, about “adequate efficacy” from a single AZN dose:
While the U.S.-based trial of the AstraZeneca vaccine is testing two doses given four weeks apart, studies the company conducted elsewhere gave some participants the two doses at intervals of six to eight weeks, or nine to 11 weeks, and some received the doses at an interval greater than 12 weeks.
So the U.K. is proceeding with the “thinly spread” vaccine experiment on its population, and we’ll see via the resulting observational study, what the single dose AZN efficacy really is like – from the infection rates and disease severity experienced by those who get infected in the ~3month window while they are waiting to get the delayed booster.
Half dose regimens while staying with phase 3 booster schedules:
Meanwhile, U.S. virologists like Dr. Fauci, Moncef Slaoui and Dr. Paul Offit are taking different sides on whether it’s a good idea to delay booster shots. Some, like Fauci, think it is a mistake. As an alternative, they are investigating what would happen to efficacy if two half doses were given, or maybe a half dose followed by a full dose booster – but sticking to the nominal booster schedule (3-4 weeks after 1st dose) that was validated in Moderna’s and Pfizer’s phase 3 trials. This approach, like the U.K. approach with AZN’s vaccine, would double the number of people who could receive the vaccine, hopefully without sacrificing the demonstrated big jump in efficacy that comes with the booster. Their analysis and deliberations are covered in a NYT article, “Scientists are studying if the Moderna vaccine supply can be doubled by cutting doses in half.” NYT, 5 Jan 2021.
From the NYT article:
The prospect of doubling the supply of Moderna doses was first raised on Sunday [3 Jan 2021] by Dr. Moncef Slaoui, the head of Operation Warp Speed, who said on the CBS program “Face the Nation” that data from Moderna’s clinical trials demonstrated that people between the ages of 18 and 55 who received two 50-microgram doses showed an “identical immune response” to the two 100-microgram doses.
That is true, said both Dr. Mascola and Dr. Anthony S. Fauci, the director of the National Institute for Allergy and Infectious Diseases, which includes the vaccine research center. But Dr. Slaoui also went one step farther, and said federal officials and Moderna were discussing possibly halving each of Moderna’s two doses — a remark that prompted pushback from the Food and Drug Administration, which would have to approve any change in the dosing regimen.
The finding Dr. Slaoui cited came from an early Phase II clinical trial, which involved hundreds of people and was designed to test only for immune response, and not for the effectiveness of the vaccine, Dr. Fauci said. It compared the immune response in people given 50 micrograms against those given 100 micrograms.
The FDA has reacted to this public debate among experts with a statement published on their website: “FDA Statement on Following the Authorized Dosing Schedules for COVID-19 Vaccines.” 4 Jan 2021.
From the FDA statement:
We have been following the discussions and news reports about reducing the number of doses, extending the length of time between doses, changing the dose (half-dose), or mixing and matching vaccines in order to immunize more people against COVID-19. These are all reasonable questions to consider and evaluate in clinical trials. However, at this time, suggesting changes to the FDA-authorized dosing or schedules of these vaccines is premature and not rooted solidly in the available evidence. Without appropriate data supporting such changes in vaccine administration, we run a significant risk of placing public health at risk, undermining the historic vaccination efforts to protect the population from COVID-19.
The available data continue to support the use of two specified doses of each authorized vaccine at specified intervals. For the Pfizer-BioNTech COVID-19 vaccine, the interval is 21 days between the first and second dose. And for the Moderna COVID-19 vaccine, the interval is 28 days between the first and second dose.
Conclusion:
At present, all of these alternative dosing protocols are irrelevant in the U.S., because the limitation on how many can be vaccinated is not due to a shortage of vaccine. The problem remains that many state and local PH authorities are having difficulty distributing and administering the doses they already have. Oh, and some people, including healthcare workers, are refusing to take them.
Stay safe,
BGL
More From Gregg Dieguez ~ “InPerspective”

Mr. Dieguez >> is a semi-successful, semi-retired MIT entrepreneur who causes occasional controversy on the Coastside, and is now a candidate for the MCC. He lives in Montara. He loves to respond to comments.
<< Mr. Laird is another semi-successful, semi-retired MIT entrepreneur but with more degrees, living in Los Altos. They roomed and caused trouble together back in the day.






Rude Bruce